Introduction
Hornets, the heavyweight champions of the wasp world, are renowned not only for their imposing size and potent sting but also for their intriguing ability to fly at night, a stark contrast to their smaller wasp relatives. This nocturnal behavior has captivated observers, yet surprisingly, it has been the subject of only limited scientific inquiry. While most smaller wasps retreat with the setting sun, hornets are often seen buzzing about in the twilight and even under the cloak of night. This raises a fascinating question: can wasps fly at night, and if so, how do they manage this feat in low-light conditions?
Initial studies, such as that by Blackith (1958), highlighted this difference, noting that European hornets (Vespa crabro) commence activity earlier in the morning and conclude later in the evening compared to common wasps like Vespula rufa, V. germanica, and V. vulgaris. Hornets were observed flying in light levels approximately 100 times dimmer than those tolerated by other wasps. Blackith proposed that moonlight, especially from a full or three-quarter moon, provides sufficient illumination for hornet flight, suggesting light intensity as the primary constraint on their nocturnal activity. More recent research by Spiewok and Schmolz (2011) further supported this, demonstrating that tethered hornets reduce their flight speed in lower light conditions (0.5 lux) compared to brighter light (850 lux).
In contrast to the limited research on hornet night flight, the visual systems and visually guided behaviors of other nocturnal and crepuscular wasps, bees, and ants have been extensively studied. These studies often point to light levels and the sensitivity of insect apposition compound eyes as limiting factors for nocturnal activity, as Kelber et al (2006) concluded for crepuscular bees, echoing Blackith’s findings for hornets.
The apposition compound eye, common among insects, consists of numerous ommatidia, each optically isolated to prevent light scatter. This design, while effective in bright light, typically limits photon capture due to the small facet lenses, seemingly making it less suitable for nighttime vision. However, some insects, including crickets, grasshoppers, cockroaches, and certain hymenoptera, utilize apposition eyes even in dim light.
Investigations into nocturnal hymenoptera like the bee Megalopta genalis, the paper wasp Apoica pallens, nocturnal bull ants, and the carpenter bee Xylocopa tranquebarica revealed common adaptations: enlarged ocelli and compound eyes featuring large facet lenses and wide rhabdoms. These adaptations enhance light sensitivity by approximately 30-fold compared to diurnal counterparts. Despite these enhancements, the light levels at which these insects navigate and even discern color are millions of times dimmer than daylight, suggesting the involvement of additional neural mechanisms such as spatial and temporal pooling.
Neural pooling has been previously suggested in European honeybees, which are diurnal, although their African subspecies, A. m. adansonii, and other species like the Asian giant honeybee, A. dorsata, and the Asian carpenter bee X. tenuiscapa, can forage on moonlit nights. Intriguingly, these species lack the specialized optical and anatomical adaptations found in obligate nocturnal bees.
This leads us back to the initial questions about hornets. What adaptations, if any, allow hornets, primarily diurnal insects, to fly at night? How frequently do they engage in nocturnal flight in naturally dark environments? To address these questions, this study examined the eyes and ocelli of the European hornet (Vespa crabro) and the common wasp (Vespula vulgaris) and collected observational data on their nocturnal flight activity.
Materials and Methods
Animals and Nest Observations
European hornets (Vespa crabro) and common wasps (Vespula vulgaris) for optical and anatomical analysis were collected in southern Sweden in September 2006. Nest observations were conducted at three different nests. One nest, located at Ammersee in southern Germany, was monitored with a video camera at its entrance during August and September 2007. Due to its small size, data from this nest primarily served to corroborate findings from other sites. A second nest in southern Sweden (55° 48′ N, 14° 3′ E) was observed on the evening of August 30th, 2008, with simultaneous light measurements using an International Light IL1700 radiometer. The third nest, also in southern Sweden (56° 40′ N, 14° 30′ E), was filmed at its entrance over four nights in August 2009 and during daylight hours across 10 days in August and September 2009. Hornet activity was quantified by counting the number of hornets entering or leaving the nest in 5-minute intervals, continuously recorded from 30 minutes before sunset to 30 minutes after sunrise. For daylight hours, 5-minute intervals were analyzed hourly.
Histology
For histological studies using light and transmission electron microscopy (TEM), hornet and wasp eyes were dissected from the head and ventrally opened to facilitate fixative penetration. Ocelli were dissected as a unit with underlying tissue attached. Samples were fixed overnight in a solution of 2% paraformaldehyde, 2% glutaraldehyde, 2% sucrose in 0.15 molar sodium cacodylate buffer, rinsed in buffer, postfixed in 1% OsO4 for one hour, dehydrated through a graded alcohol series, and embedded in Epon.
For TEM, ultrathin transverse sections of the eye were stained with 2% uranylacetate and lead citrate and examined using a Jeol 1230 transmission electron microscope (Tokyo, Japan). For light microscopy, 3 µm longitudinal sections of both eyes and ocelli were stained with Azur II-Methylenblue and photographed with a Zeiss light microscope equipped with an Olympus digital camera.
For scanning electron microscopy, entire heads preserved in 70% ethanol were dehydrated, air-dried, and gold-coated. Micrographs were taken using a Jeol LV 5600 SEM (Tokyo, Japan). Body size was assessed by measuring the intertegular span, the thorax width between the wing bases (tegulae). Head width and length were measured for comparison with existing data.
Eye Maps
Interommatidial angle maps were generated only for Vespa crabro as a pseudopupil could not be observed in Vespula vulgaris. The method, detailed in previous publications, involves immobilizing the wasp, positioning its head at the center of curvature of a Leitz goniometer, and aligning its eyes. The goniometer allowed for precise tilting in latitude and longitude. The wasp’s eyes were illuminated using orthodromic illumination, making the pseudopupil visible. Pictures were taken at 10° intervals across longitudinal and latitudinal positions, limited to the frontal eye region due to experimental constraints. BaSO4 dust landmarks aided in tracking pseudopupil positions. Coordinates of the facet at the pseudopupil center were determined from each photograph, and interommatidial angles and facet diameters were calculated and plotted on a sphere representing the visual space.
Focal Length
Focal length and back focal distance were determined using a modified hanging drop method. Small corneal pieces from the fronto-ventral eye region were dissected, cleaned, and placed in a saline drop on a coverslip, external side facing outwards. This was inverted over a microscope slide with an o-ring and petroleum jelly seal, and mounted on a Nikon light microscope. Striped patterns of known size were placed over the microscope lamp aperture, and the facet-formed images were photographed. Focal length was calculated using equation (1), relating object and image spatial wavelengths and object distance. Back focal distance was measured by focusing on debris on the cornea back and then the focal plane, measuring the distance with a micrometer gauge and correcting for saline refractive index. The same procedure was applied to lateral and medial ocelli.
F-number and Optical Sensitivity to White Light
The F-number, a measure of lens light-gathering ability, was calculated as the ratio of focal length (f) to corneal facet diameter (D) (equation 2). Optical sensitivity (S), representing the light absorbed by a photoreceptor viewing an extended white light source, was calculated using a simplified formula (equation 3) incorporating facet diameter (D), acceptance angle (Δρ), rhabdom length (l), and absorption coefficient (k). The acceptance angle (equation 4) was estimated as the ratio of distal rhabdom diameter (d) to focal length (f).
Results
Flight Activity of Vespa crabro
Flight activity observations during late summer in southern Sweden, where twilight lasts approximately three hours, showed consistent hornet activity during daylight hours, from about one hour pre-sunrise to one hour post-sunset, at light intensities of 1 cd/m2 or higher. An average of 15 hornets were observed entering or leaving the nest every five minutes, with a slight increase in activity during early evening hours (Fig. 1A, B). As light intensity decreased below this level, down to 0.001 cd/m2, flight activity diminished (Fig. 1C, D). Hornet departures and arrivals were rare at even dimmer light levels.
Figure 1. Flight activity of Vespa crabro. Data were recorded in Sweden, at 56°40′ North and 14°30′ East, during 4 nights between 14/8/2009 and 19/8/2009, and during 10 days in August and September 2009.
A, B Examples of flight activity during two 24 hour periods. During the day, the number of bees entering (open squares) and leaving (crosses) the nest within five minutes was recorded every hour, for several hours per day. During the night, these numbers were recorded continuously. Dark grey shading indicates periods with no sunlight (sun more than 18° below the horizon), medium grey indicates time when the sun is between 12° and 18° below the horizon, and light grey shading indicates times when the sun is between 0° and 12° below the horizon. The moon was a waning crescent, and the moon symbols indicate when it was visible. C, D The average number (and standard deviation) of hornets entering (black bars) and leaving (light grey bars) the nest within five minutes, during dawn (C) and dusk (D) is given as a function of light intensities. Moonlight is in the range between 0.0001 and 0.01 Cd/m2 from a new moon to a full moon, depending on the elevation. Data from four nights and ten days.
Eyes
Vespa crabro workers are significantly larger (4.8 mm intertegular span) than Vespula vulgaris workers (2.5 mm intertegular span), a size difference reflected in head and eye dimensions (see Fig. 2A, B and Table 1). V. crabro head length is 6 mm, head width 5.6 mm, and eye length 3.7 mm, compared to V. vulgaris values of 3.2 mm, 3.2 mm, and 2.2 mm, respectively. Despite their larger absolute size, hornet eyes are proportionally smaller relative to their body and head size than those of common wasps.
Figure 2. The eyes of Vespa crabro and Vespula vulgaris (left and right side, respectively, in each pair of figures).
Each pair of figures has the same scale. A, B Frontal view of the head, note the cuticular indentation into the eye, scale bar 1 mm. C, D Longitudinal section through several ommatidia in the eye, showing a thick cornea, the crystalline cone, and the rhabdoms, scale bar 50 µm. E, F Facets of the compound eye, scale bar 50 µm. G, H Cross sections through the distal rhabdom, scale bar 0.5 µm.
Table 1. Body and eye measurements of four species of Vespidae.
| Symbol | Unit | Vespa crabro | Vespula vulgaris | Apoica pallens1 | Polistes occidentalis1 |
|---|---|---|---|---|---|
| Intertegular width | mm | 4.8±0.1 | 2.5±0.1 | 2.7±0.1 | |
| Head length | mm | 6.0 | 3.2 | 3.1 | 3.7 |
| Head width | mm | 5.6 | 3.2 | 3.5 | 4.0 |
| Eye length | mm | 3.7±0.1(3.92) | 2.2±0.1(2.32) | 3.6 | 3.5 |
| Maximum corneal facet diameter | D | µm | 35.2±2.4 | 27.0±2.9 | 26 |
| Corneal thickness | µm | 110 | 50 | 60 | 80 |
| Crystalline cone length | µm | 60 | 40 | 40 | 60 |
| Distal rhabdom diameter | d | µm | 2.1 | 2.4 | 8 |
| Rhabdom length | l | µm | 370 | 240 | 300 |
| Ommatidial length | µm | 540 | 330 | 400 | 490 |
| Focal length | f | µm | 110±16 | 67±4 | 64±4 |
| F-number | F | 3.1 | 2.5 | 2.5 | 3.2 |
| Theoretical acceptance angle | Δρ | ° | 1.0 | 2.1 | 7.2 |
| Optical sensitivity | S | µm2sr | 0.14 | 0.23 | 3.0 |
External measurements are from 4 V. crabro and 4 V. vulgaris.
1data from Greiner 2006, except the intertegular width that was measured for this study, from 3 animals in the authors’ collection.
2data from Blackith 1958.
V. crabro ommatidia are 540 µm long, featuring a notably thick cornea (110 µm), a 60 µm crystalline cone, and a 370 µm rhabdom (Fig. 2C). The distal rhabdom diameter (d) is 2.1 µm (Fig. 2G), and the focal length (f) is 110±16 µm. Corneal facet diameter (D) ranges from 35 to 36 µm in the fronto-ventral eye region (Fig. 2E, Fig. 3A, C), decreasing towards the lateral, dorsal, and ventral edges.
Figure 3. Contour maps of facet diameters D (A, C) and interommatidial angles Δϕ (B, D) in two Vespa crabro worker eyes.
The thick vertical lines indicate the frontal visual field borders, anterior corresponds to latitude and longitude of 0°, dorsal to a latitude of +90°, ventral to a latitude of −90°, and lateral to a longitude of +90°. Facet diameters and interommatidial angles are indicated as isolines. In both animals, the ommatidia with the largest facet diameters (36 µm) also have the smallest interommatidial angles (1°) and look into a frontal and slightly ventral direction. The bold horizontal lines indicate the region in the visual field around +10° that is seen by ommatidia above and below the cuticular indentation into the hornet eye. Interommatidial angles in this region cannot be determined as accurately as in other eye regions.
For Vespula vulgaris (Figure 1, Table 1), ommatidial length is 330 µm with a 50 µm cornea, 40 µm crystalline cone, and 240 µm rhabdom. Distal rhabdom diameter is 2.4 µm, and focal length is 67±4 µm. Corneal facet diameter in the fronto-ventral eye region is 27 µm.
Calculated F-numbers are 3.1 for V. crabro and 2.5 for V. vulgaris. Theoretical acceptance angles (Δρ) for fronto-ventral ommatidia are 1.0° for V. crabro and 2.1° for V. vulgaris. In V. crabro, acceptance angles match interommatidial angles (Δϕ) in the frontal eye region (Fig. 3C, D).
Hornets, like other vespids, possess a frontal indentation in their eyes (Fig. 1A, B), causing a small visual field region around 10° elevation to be viewed twice. Measurements within the ventral eye region were more reliable, revealing that the area with the smallest interommatidial angles, indicating highest potential resolution, coincides with the area of largest and most sensitive ommatidia, suggesting an acute zone.
Optical sensitivity (S) in this eye region is 0.14 µm2sr for V. crabro and 0.23 µm2sr for V. vulgaris. Surprisingly, V. crabro eyes are optically less sensitive than V. vulgaris eyes, contradicting expectations based on their nocturnal flight activity.
Ocelli
The three ocelli of V. crabro and V. vulgaris are located centrally on the head, between the compound eyes (Figs 1, 4). Minor size differences exist between lateral and medial ocelli in both species. V. crabro medial ocellus diameter is 359 µm, lateral ocelli 336 µm, larger than V. vulgaris medial (207 µm) and lateral (211 µm) ocelli, but proportionally similar relative to body or head size (Table 2).
Figure 4. Ocelli of Vespa crabro (left side) and Vespula vulgaris (right side).
Scales in each pair of figures are the same for both species. A, B Arrangement of all three ocelli on the head of the wasp, scale bar 200 µm. C, E Sagittal sections through the medial ocellus, scale bar 100 µm. D Close-up of the medial ocellus of V. vulgaris, scale bar 100 µm. F, G Focal length measured in hanging drop preparations superimposed on sketches of the medial ocelli indicating the lens (l) and retina (r) show that the focal plane lies within the retina. Same scale as C and E.
Table 2. Ocellar size in four species of Vespidae.
| Symbol | Unit | Vespa crabro | Vespula vulgaris | Apoica pallens1 | Polistes occidentalis1 |
|---|---|---|---|---|---|
| Ocellar diameter | |||||
| Lateral | D | µm | 336 | 211 | 414 / 486 |
| Medial | D | µm | 359 | 207 | 416 / 427 |
| Ocellar diameter/head width | |||||
| Lateral | 0.060 | 0.066 | 0.117 | 0.056 | |
| Medial | 0.064 | 0.065 | 0.120 | 0.070 | |
| Focal length | |||||
| Lateral | f | µm | 292±74 | 149±10 | 331 / 450 |
| Medial | f | µm | 246±6 | 140±23 | 357 / 436 |
| Back focal distance | |||||
| Lateral | L | µm | 178±19 | 107±22 | 184 / 304 |
| Medial | L | µm | 145±19 | 104±19 | 162 / 190 |
Data from 5 Vespa crabro and 2 Vespula vulgaris are from this study. 1 Data from Warrant et al 2006. Ocelli of Apoica pallens and Polistes occidentalis are astigmatic and the values for the long and short axes are given.
V. crabro ocellar focal lengths were 292±74 µm (lateral) and 246±6 µm (medial). V. vulgaris focal lengths were 149±10 µm (lateral) and 140±23 µm (medial). Optical back focal distances for V. crabro were 178±19 µm (lateral) and 145±19 µm (medial); for V. vulgaris, 107±22 µm and 104±19 µm, respectively. Image focusing within the retina is indicated when these distances are superimposed on ocelli longitudinal sections (Fig. 4G, H).
Discussion
Hornet and Wasp Flight Activity
Our findings corroborate Blackith’s (1958) observations, confirming that European hornets, Vespa crabro, exhibit flight activity in dim twilight, down to light intensities between 0.01 and 0.1 cd/m2, extending their activity beyond civil twilight but not as late as nautical twilight (Fig. 1A, B). This suggests hornets can indeed fly on nights with significant moonlight, as Blackith proposed. Common wasps, in contrast, are restricted to light intensities approximately one hundred times brighter, limiting their flight to the brighter phase of civil twilight. While Blackith attributed the activity differences primarily to light intensity, we acknowledge that temperature or other factors like food availability might also play a role in the observed flight activity.
Hornet Eyes Lack Specific Adaptations for Dim Light Vision
The clear difference in nocturnal flight capability between hornets and common wasps is surprisingly not mirrored by specialized adaptations in their eyes. In fact, hornet eyes exhibit slightly lower optical sensitivity compared to wasp eyes. The optical sensitivity of both species is comparable to that of the diurnal wasp Polistes occidentalis. Vespa crabro eyes, while featuring larger facet lenses and longer focal lengths, result in F-numbers similar to those of diurnal species. Even Apoica pallens, an exclusively nocturnal wasp, has a similar F-number. The enhanced sensitivity of A. pallens eyes stems primarily from their exceptionally wide rhabdoms (8 µm), resulting in large acceptance angles. In comparison, V. vulgaris has acceptance angles of 2°, and V. crabro even smaller angles, as low as 1°.
High Resolution and Night Vision: The Advantage of Size
The optical sensitivity observed in Vespa crabro (0.14 µm2sr) is comparable to diurnal bees (Apis mellifera), humans, and large bee species capable of moonlit foraging like A. dorsata and X. tenuiscapa. These species also share narrow rhabdoms and small acceptance angles with V. crabro. A common trait among these species is their larger body size, leading to larger eyes. Larger eyes accommodate more ommatidia and larger facet lenses, contributing to both higher resolution and increased sensitivity. In dim-light adapted species, sensitivity is often prioritized over resolution.
Studies on bees foraging in extremely low light conditions, such as M. genalis in rainforest twilight (0.0001 cd/m2) and X. tranquebarica on moonless nights (0.00001 cd/m2), revealed only a 30-fold increase in eye sensitivity compared to diurnal bees, despite small interommatidial angles. This apparent paradox, where high resolution coexists with dim-light activity, suggests neural spatial summation as a mechanism to enhance sensitivity at the cost of resolution. This solution might not be optimal for obligate nocturnal animals but is plausible in the context of evolutionary transition from crepuscular to nocturnal behavior.
Larger eyes, associated with larger body size, are crucial for leveraging spatial pooling without significant resolution loss. This adaptation likely allowed larger bees and hornets to exploit resources unavailable to smaller insects during daylight hours. Spatial pooling is hypothesized to be an initial adaptation to dim-light vision, enabling insects to maintain high spatial resolution during daylight and enhance sensitivity in dim light. Only in truly nocturnal species like X. tranquebarica and M. genalis did further evolution favor widened rhabdoms, sacrificing high resolution for even greater light sensitivity, allowing flight in extremely dark conditions. Our hornet study supports this hypothesis, suggesting that A. pallens‘s obligate nocturnal behavior and wide rhabdoms may have evolved through a facultative twilight activity stage similar to that of V. crabro. Like crepuscular bees, V. crabro ommatidia possess relatively low optical sensitivity, implying reliance on spatial and/or temporal summation for dim-light flight, potentially sacrificing spatial and temporal resolution at night. The exact extent of pooling in hornets remains to be investigated.
Behavioral evidence for temporal pooling comes from observations that tethered hornets, similar to nocturnal bees, fly slower in dim light. Further research is needed to explore hunting behavior, flight control, and optic pathway information processing in hornets. Future studies will measure spatio-temporal resolution in hornets and wasps at varying light levels. Honeybees are the only species where a relationship between spatial resolution and light intensity, indicating pooling, has been clearly demonstrated.
Research on the crepuscular butterfly Caligo memnon suggests that the evolution of crepuscular and nocturnal vision in apposition-eyed insects with wide rhabdoms began with larger body and eye size, followed by the development of wide rhabdoms. Facultatively nocturnal bees and wasps appear to be in an earlier stage of this evolutionary path. Our data suggest neural summation precedes the evolution of wide rhabdoms in hymenoptera transitioning to nocturnal lifestyles, and further discovery of intermediate forms is anticipated.
Beyond eye size, a larger body size also confers the advantage of better temperature regulation. Future research will examine body temperatures of flying wasps and hornets to understand the role of body size in maintaining flight-suitable temperatures.
Wasp Ocelli: Potential for Spatial Resolution?
Previous studies have linked ocellar size to dim-light activity in hymenoptera, with nocturnal species having larger ocelli. While V. crabro ocelli are large, they are not as proportionally large as those of A. pallens and are more similar in relative size to diurnal wasps. This suggests V. crabro ocelli are not specifically adapted for dim light but are simply larger due to larger body size.
However, like other wasps studied (A. pallens and P. occidentalis), V. crabro and V. vulgaris ocelli have relatively short focal lengths, focusing images within the retina (Fig. 4). This contrasts with bees and many other insects where underfocused ocelli are thought to primarily measure general light intensity. The focused ocelli in wasps theoretically allow for some retinal resolution, similar to dragonflies, the only insects currently known to utilize ocellar resolving power to form coarse images. Further investigation into the anatomy and physiology of ocellar photoreceptors and interneurons is needed to confirm this in wasps.
Conclusions
This study confirms that European hornets, Vespa crabro, can indeed fly at night, specifically in dim twilight conditions down to 0.001 cd/m2, enabling flight even under bright moonlight. This nocturnal capability exists despite the absence of typical optical or anatomical adaptations in their eyes and ocelli seen in truly nocturnal hymenoptera. Instead, their visual systems are remarkably similar to diurnal wasps. Analogous to facultatively nocturnal bees, we propose that neuronal summation is the key mechanism enabling hornet flight in dim light. This suggests that neuronal summation is a crucial initial step in the evolution towards nocturnal lifestyles in hymenopteran insects, preceding anatomical adaptations like widened rhabdoms.
Acknowledgments
We extend our gratitude to Dan and Maria Nilsson, as well as Torgny and Ruth Kornfeldt, for their generous access to hornet nests in their gardens, and to Eva Landgren, William Sidemo-Holm, and Caroline Sollevi for their valuable assistance with data analysis. We also thank Dr. Elmar Billig for sharing his video recordings of a hornet nest in southern Germany.
Footnotes
Competing Interests: The authors declare no competing interests.
Funding: This research was supported by the Crafoord Foundation and the Swedish Research Council (grants 2003-7165, 2006-4510). The funding sources had no involvement in the study design, data collection, analysis, publication decisions, or manuscript preparation.
References
[1] Blackith RE (1958) The ecology of Vespa vulgaris L. in unwooded habitats. J Anim Ecol 27: 371–391.
[2] Spiewok S, Schmolz E (2011) Influence of light intensity on the flight speed of tethered hornet workers (Vespa crabro). J Insect Physiol 57: 833–838.
[3] Warrant EJ, Kelber A, Gribakin FG, Land MF (2004) Eye design in nocturnal bees and wasps: matching optics to visual tasks. In: Warrant E, Nilsson D-E, editors. Invertebrate Vision. Cambridge: Cambridge University Press. pp. 205–240.
[4] Greiner B (2006) Vergleichende Untersuchungen zur Retina von nachtaktiven und tagaktiven Hautflüglern (Hymenoptera). PhD thesis. University of Basel.
[5] Kelber A, Eigner M, Pfaff M, Warrant EJ (2006) Scotopic colour vision in the nocturnal bee Megalopta genalis. J Exp Biol 209: 687–693.
[6] Greiner B, Ribi WA, Warrant EJ (2007) Retinal and optical adaptations for nocturnal vision in the Australian bull ant Myrmecia pyriformis. Cell Tissue Res 328: 325–338.
[7] Somanathan H, Borges RM, Warrant EJ, Kelber A (2008) Nocturnal and diurnal foraging in the carpenter bee Xylocopa tenuiscapa (Apidae): visual adaptations and colour preferences. J Comp Physiol A 194: 585–594.
[8] Somanathan H, Kelber A, Warrant EJ (2009) Spatial vision in the giant honeybee Apis dorsata: acuity, contrast sensitivity and resolution limit. J Exp Biol 212: 1541–1549.
[9] Narendra A, Reid SF, Hemmi JM, Zollikofer CP, Schleidt WM (2011) Caste-specific visual adaptations in ants. Proc R Soc B 278: 1541–1548.
[10] Greiner B, Warrant EJ (2013) Nocturnal insects see in colour. Phil Trans R Soc B 368: 20120471.
[11] Warrant EJ, Gribakin FG, Land MF (2006) Ocellar optics in nocturnal and diurnal wasps. J Comp Physiol A 192: 549–562.
[12] Somanathan H, Zeil J, Kelber A, Warrant EJ (2008) Navigation under the stars: insect ocelli and nocturnal way-finding. Phil Trans R Soc B 363: 3553–3565.
[13] Theobald JC, Warrant EJ, O’Carroll DC (2007) The spatial resolving power of the nocturnal bee Megalopta genalis. J Comp Physiol A 193: 1213–1223.
[14] Warrant EJ, Davies NW, Nilsson D-E, Meyer-Rochow VB (1996) Mechanisms for maximizing sensitivity and resolution in insect superposition eyes. In: Land MF, Nilsson D-E, editors. Animal Eyes. Oxford: Oxford University Press. pp. 290–318.
[15] Spaethe J, Tautz J (2003) Behavioural and morphological caste differences in the honeybee queen (Apis mellifera ligustica). Insectes Soc 50: 3–10.
[16] Land MF (1997) Visual acuity in insects. Annu Rev Entomol 42: 147–177.
[17] Homann H (1924) Beiträge zur Kenntnis der Facettenaugen der Dipteren. Z wiss Zool 123: 267–320.
[18] Kirschfeld K (1974) The absolute sensitivity of photodetectors based on the principle of energy transfer. Biophys Struct Mech 1: 339–344.
[19] Land MF (1981) Optics of invertebrate eyes. In: Autrum H, editor. Handbook of Sensory Physiology, vol VII/6C. Berlin: Springer. pp. 471–592.
[20] Warrant E, Nilsson D-E (1998) Invertebrate vision. Cambridge: Cambridge University Press.
[21] Bruno M, Nilsson D-E, Warrant E (2001) Anatomical and optical adaptations for nocturnal vision in a dung beetle. J Comp Physiol A 187: 293–304.
[22] Stavenga DG (1979) Pseudopupils of compound eyes. In: Autrum H, Jung R, Loewenstein WR, MacKay DM, Perl ER, editors. Handbook of Sensory Physiology, vol VII/6A. Berlin: Springer. pp. 357–439.
[23] Stavenga DG, Kuiper JW (1977) Insect pupil mechanisms. II. Pigment migration in the retinula cells of Heliothis and Pieris. J Comp Physiol 113: 55–72.
[24] Kelber A, Vorobyev M (2006) Colour vision and colour constancy in invertebrate animals. Phil Trans R Soc B 361: 293–304.
[25] Theobald JC, O’Carroll DC, Warrant EJ (2008) Temporal summation in the nocturnal bee Megalopta genalis. J Comp Physiol A 194: 757–767.
[26] Frederiksen R, Warrant E (2008) Crepuscular and nocturnal vision in butterflies. J Exp Biol 211: 3787–3795.
[27] Kerfoot BA (1967) Ocellar size in bees. J Kans Entomol Soc 40: 23–28.
[28] Mizunami M, Yamashita T, Yokohari F, Tateda H (1982) Spectral responses of ocellar units in the honeybee. J Exp Biol 99: 415–423.
[29] Stange G, Stowe S, Chahl J, Wirz-Justice A, Nilsson D-E (2002) Ocellar mediation of rapid steering in dragonflies. J Exp Biol 205: 1239–1249.
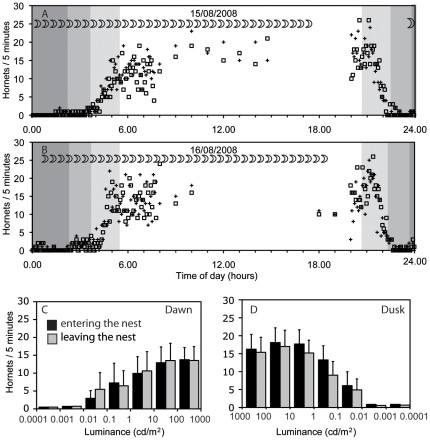
 Figure 2
Figure 2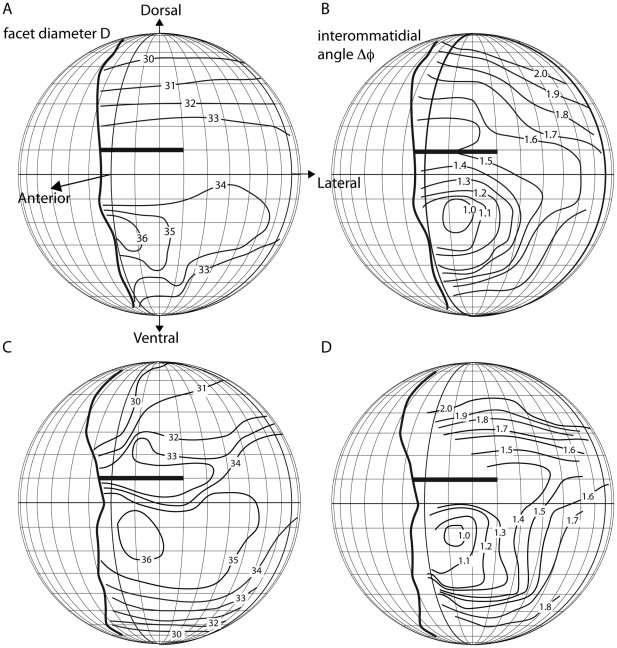 Figure 3
Figure 3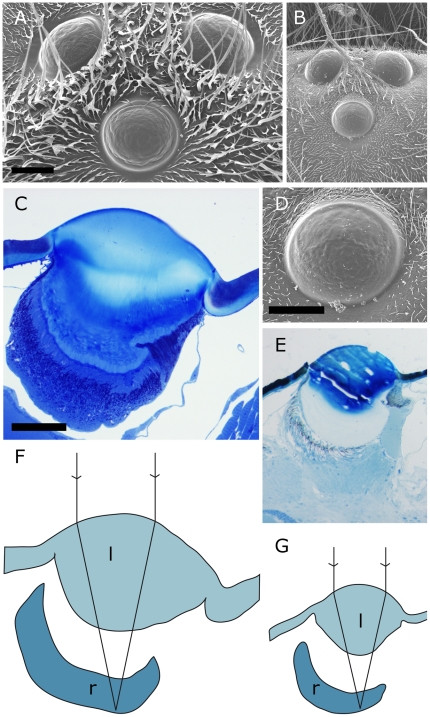 Figure 4
Figure 4