Bar-headed geese are renowned for their incredible migrations across the Himalayas, a feat of endurance at extreme altitudes. These birds undertake one of the most iconic high-altitude migrations globally. During these journeys, their bodies face significant challenges as heart rates and metabolic costs escalate with increasing elevation, often reaching near-maximal levels during steep ascents. Their remarkable ability to sustain flight under such oxygen-deprived conditions hinges on the unique cardiorespiratory physiology inherent to birds, coupled with specific evolutionary adaptations that enhance oxygen transport throughout their bodies.
“On one cold and still night in early April, I stood beside the Barun glacier [near Mount Makalu, the fifth highest mountain in the world at 8,463 m above sea level] . . . Coming from the south, the distant hum became a call. Then, as if from the stars above me, I heard the honking of bar-headed geese.”–Lawrence Swan (46)
The migration of bar-headed geese has captivated scientists and nature enthusiasts alike since early mountaineers first witnessed them traversing the Himalayan peaks. These geese spend their summers breeding from Mongolia to the Tibetan Plateau. As autumn approaches, most embark on long southward flights to the Indian subcontinent, returning north in the spring (24, 47). Flight, the most energy-demanding form of movement for vertebrates, requires an exceptionally high rate of oxygen consumption (51). However, the air at high altitudes in the Himalayas contains dramatically less oxygen—only one-third to one-half of what’s available at sea level. This poses a fascinating paradox: how can bar-headed geese meet the intense oxygen demands of flight in such thin air? This article delves into the evidence confirming this incredible feat and explores the physiological mechanisms that enable high-altitude flight in these avian champions. We will examine recent research into the physiological ecology of their natural migration and the unique respiratory and metabolic adaptations that solve the puzzle of their high-altitude capabilities.
The Extreme Altitude Flight Challenge for Geese
Bar-headed geese navigate altitudes that present extreme challenges even to seasoned human mountaineers and other animals adapted to lower elevations. Satellite tracking has shown geese migrating between India and Mongolia routinely crossing the Himalayas across a wide front (47) (FIGURE 1). The majority of these birds ascend to altitudes between 5,000 and 6,000 meters (approximately 16,400 to 19,700 feet) during their migration. At these elevations, the partial pressure of oxygen (Po2) is roughly half that at sea level. Remarkably, they occasionally fly even higher; one recorded bird reached an astonishing 7,290 meters (nearly 24,000 feet) (16, 24, 47). While some anecdotal accounts, often based on auditory or visual sightings, suggest even greater heights above the highest Himalayan peaks (over 8,000 meters or 26,200 feet), where Po2 is a mere third of sea-level values (46), these observations are less scientifically verified (16). Even at the lower, confirmed altitudes, the level of hypoxia is significant enough to substantially reduce maximal oxygen uptake rates in humans (53). In fact, the oxygen levels at the summits of the highest Himalayan peaks are considered barely sufficient to sustain basic human metabolism (53).
Therefore, bar-headed geese face the immense challenge of maintaining the high rates of oxygen consumption necessary for flapping flight—which can be 10 to 15 times their resting levels during steady flight at sea level wind tunnels (51)—in an atmosphere that severely restricts aerobic metabolism for many lowland creatures (49). Adding to these challenges, high altitudes often bring extremely low temperatures, well below freezing year-round in the high Himalayas (56). This could necessitate additional metabolic energy for thermogenesis if the heat generated from exercise isn’t sufficient to maintain their body temperature. Furthermore, maintaining water balance during these flights is a significant hurdle in the dry, high-altitude air, as water loss can limit flight duration even at sea level for some bird species (11).
FIGURE 1.
Figure 1: Bar-headed geese migration routes across the Himalayas. (A) Satellite tracking data showing migration paths of bar-headed geese from Mongolia to India, crossing the Himalayas. Elevation is indicated by colored shading, and white crosses mark the highest peaks (above 8,000m). (B) Steep and rapid ascent over the mountains during northward migration from India, with climb rates between 0.8–2.2 km/h. Adapted from Ref. 16.
As bar-headed geese ascend, the decreasing air density makes generating lift increasingly difficult. Physiological data logged during migration reveals that average heart rates during flight increase with altitude (FIGURE 2B). Geese also spend more time flying with heart rates nearing their maximum capacity when above 4,800 meters (roughly 15,750 feet) (4). Whenever possible, these geese mitigate the metabolic demands of high-altitude flight by choosing lower routes, such as river valleys, or by leveraging orographic lift or katabatic winds near mountains (4, 16) (FIGURE 2C). Despite these strategies, bar-headed geese primarily rely on flapping flight and rarely glide, even during steep descents (4).
FIGURE 2.
Figure 2: Metabolic cost of high-altitude goose flight indicated by heart rates during migration. (A) Altitude and heart rate fluctuations of a bar-headed goose over the Tibetan Plateau, showing heart rate changes during ascent and descent. (B) Average flight heart rates increase with elevation. (C) Environmental aids like uplifting winds can reduce heart rates and metabolic costs during climbing. The example shows assisted lift (blue lines) outside the typical ascent rate-heart rate relationship (red indicates observation density). Adapted from Ref. 4.
Crossing the Himalayas from India to the Tibetan Plateau requires bar-headed geese to endure prolonged periods of ascent, demonstrating the longest sustained climbing flight rates recorded and potentially facing headwinds (17). Climbing flight is metabolically more demanding than level flight, generally requiring higher average heart rates and wing-beat frequencies (4). Indeed, individual geese show increased heart rates during ascent and decreased rates during descent (FIGURE 2A). A positive correlation exists between ascent rate and heart rate (FIGURE 2C) (4), suggesting that utilizing upslope tailwinds during ascent would be advantageous (7, 9). However, upslope tailwinds are most common during the day in mountainous regions, while bar-headed geese often migrate at night and in the early morning when downslope winds prevail (17). Although nighttime flights likely increase metabolic costs due to the need to flap harder against downslope winds for climbing, the darkness may offer reduced predation risks (e.g., from predatory birds), more stable and less turbulent air currents, and cooler air, which is denser and has a slightly higher Po2. These benefits might outweigh the metabolic costs of climbing at night.
Ecophysiological studies of bar-headed geese migration highlight the significant challenges of high-altitude flight. While they may utilize wind assistance when available, similar to lowland geese (9), they frequently experience extended periods of high heart rates and intense metabolic activity (4). The fact that these periods occur in hypoxic air (Po2 ≤50% of sea level) makes their physiological achievement even more remarkable. Their ability to transport sufficient oxygen to flight muscles and other tissues to sustain these high metabolic demands appears to depend on a combination of physiological traits. Some are general avian traits, acting as “high-altitude exaptations”—traits evolved for other purposes but beneficial at high altitudes. Others are specialized traits that likely evolved specifically as adaptations to high-altitude environments (FIGURE 3).
FIGURE 3.
Figure 3: Physiological traits aiding high-altitude flight in geese. (A) General avian traits (blue) and bar-headed goose specializations (orange) facilitating high-altitude flight. (B) Qualitative effects of specializations increasing oxygen tension (Po2) across the oxygen transport cascade compared to lowland geese. Capillary Po2 decreases along capillaries as blood loses O2 to tissues, resulting in a range of potential cellular Po2. Adapted from Refs. 21, 48.
Avian Physiology: Natural Advantages for High-Altitude Flight
The inherent hypoxia tolerance of birds, possibly evolving alongside enhanced cardiorespiratory performance for flight, is crucial for bar-headed geese’s high-altitude capability (12, 38). Early studies showed that even lowland sparrows could fly in wind tunnels breathing air simulating the Po2 at 6,100 meters (over 20,000 feet), an oxygen level that renders domestic mice comatose (50). Birds generally exhibit greater hypoxia tolerance than mammals. Bar-headed geese and other hypoxia-tolerant birds can survive at significantly lower Po2 levels (around 2.7 kPa) compared to the most hypoxia-tolerant euthermic mammals (like mole rats, around 4.7 kPa) (49). Several unique aspects of avian respiratory and cardiovascular physiology across the oxygen cascade (21, 48) (FIGURE 3) likely contribute to this heightened hypoxia tolerance, serving as crucial exaptations for high-altitude flight.
Birds are believed to achieve higher ventilation rates than mammals under hypoxic conditions. Hypoxemia, the decline in arterial Po2, triggers increased breathing in response to low environmental oxygen. However, this also increases CO2 excretion (42), leading to low blood Pco2 (hypocapnia). Hypocapnia can restrain the hypoxic ventilatory response and cause blood alkalosis. Yet, birds can maintain arterial Pco2 levels as low as 2 Torr without the typical impairments to cellular function seen in mammals at similar levels (35). This may stem from a superior ability to quickly restore blood pH when Pco2 changes (10) or because their brain vasculature is less sensitive to hypocapnia (discussed later). Consequently, oxygen delivery to the gas-exchange surface in hypoxic environments might be more efficient in birds compared to mammals.
The structure and function of avian lungs provide a naturally superior gas-exchange capacity compared to mammalian lungs. Birds possess a unidirectional airflow system in their lungs, originating from reptiles (14) and evolving into a highly efficient gas exchanger (36). Blood flow in pulmonary capillaries is perpendicular to airflow through parabronchi, making the bird lung a cross-current gas exchanger (28). Cross-current exchange is inherently more effective than the alveolar exchange mechanism in mammalian lungs. This allows birds in hypoxia to achieve arterial Po2 levels exceeding that of expired gas (33, 35). The capacity for oxygen diffusion in bird lungs is also exceptionally high due to their thin gas-exchange tissue (0.1–0.2 μm compared to 0.4–0.8 μm or more in mammals) and generally larger surface area (40–100 cm2/g versus 15–40 cm2/g in nonflying mammals) (52, 54.
The unique physiology of avian pulmonary vessels may also offer resistance to high-altitude pulmonary edema, a major concern in mammalian acute mountain sickness (37). Mammalian pulmonary vessels constrict in response to hypoxia, potentially leading to pulmonary hypertension, impaired gas exchange, and edema (26, 37). In contrast, avian pulmonary vasculature does not constrict under hypoxic conditions. Pulmonary arterial pressure in birds only increases in hypoxia when cardiac output rises (6, 13, 55). The avian blood-gas barrier is also considered mechanically stronger and more resistant to stress failure than that of mammals (54.
Several cardiac differences between birds and mammals support higher cardiac outputs and greater convective oxygen delivery during hypoxia. Bird hearts are approximately 50% larger and have greater stroke volumes than mammals of similar size. Birds can also sustain heart rates during free flight that are comparable to or even exceed those of mammals during maximal exercise (3, 15). Capillary density in avian cardiac muscle also appears higher than in mammals (12), suggesting a greater oxygen diffusion capacity, potentially making bird hearts more resilient to oxygen limitation under hypoxia.
The capacity for oxygen diffusion into peripheral tissues seems to be higher in birds than in mammals and other vertebrates. Capillary exchange capacity is greater in bird flight muscles (capillary length per fiber volume of 6,015 and ∼13,910 mm−2 in pigeons and hummingbirds) compared to locomotory muscles of nonflying mammals (5,700 and 1,890 mm−2 in deer mice and dogs) (29). This difference is largely due to a mesh of branching capillaries surrounding avian muscle fibers, which are smaller than those in similar-sized nonflying mammals (fiber diameters of 20 and 14 μm in pigeons and hummingbirds, and 29 and 45 μm in deer mice and dogs) (29).
Brain physiology in birds also presents differences that may protect against cerebral dysfunction in hypoxia. Unlike mammals (1), cerebral blood flow in birds is not inhibited by respiratory hypocapnia (12). This should improve brain oxygenation during environmental hypoxia, although direct measurement confirmation is still needed. Avian neurons also exhibit an inherently higher tolerance to low cellular oxygen levels (demonstrated by greater survival of duck and chicken cerebellar slices compared to rat slices after 60 minutes of anoxia) (25), indicating better protection against cellular damage from oxygen deprivation. An interesting unanswered question is whether birds experience hypoxic cerebral edema, a dangerous consequence of high-altitude exposure in humans (19).
While the unique respiratory and cardiovascular physiology of birds enhances hypoxia tolerance and exercise capacity by improving overall oxygen transport, most bird species cannot fly at extremely high altitudes. Many cannot tolerate the hypoxic conditions at the summits of the world’s highest mountains (5), and some migrate exceptionally long distances to avoid mountain ranges (20). Why bar-headed geese don’t avoid the Himalayas is unclear. One theory suggests that the species (or its ancestor) began migrating between South and Central Asia during the late Pliocene or early Pleistocene, when the Himalayas were significantly lower (46). As migration routes can be genetically ingrained, the species may have gradually evolved to fly at increasingly higher altitudes over millennia along the same routes. Regardless of the evolutionary path, modern bar-headed geese have developed specialized traits distinguishing them from most other birds, enabling them to sustain the high oxygen demands of flight in the oxygen-thin air at extreme altitudes.
Specialized Physiology of Bar-Headed Geese: Adaptations for Extreme Heights
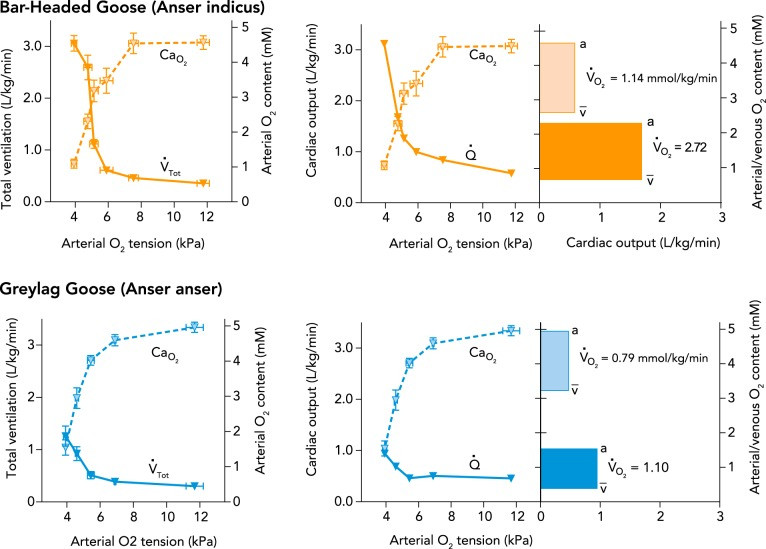
Bar-headed geese stand out from other waterfowl due to their exceptional ability to transport and utilize oxygen at high rates in hypoxic environments. They can tolerate extreme hypoxia even at rest (inhaled Po2 as low as ∼2.7 kPa, equivalent to ∼12,000 meters or nearly 40,000 feet), far surpassing the tolerance of many lowland waterfowl (5, 41). Bar-headed geese also maintain their body temperature in hypoxia down to a lower inspired Po2 (∼9 kPa) than lowland waterfowl (∼12 kPa), and experience less body temperature depression (39). Remarkably, they increase their metabolic rate two- to threefold in hypoxia at rest (inspired Po2 between ∼4 and 9 kPa), likely to support the oxygen demands of their respiratory and cardiovascular responses to the low-oxygen environment (FIGURE 4) (5, 41). Furthermore, bar-headed geese can achieve the high metabolic rates required for flight in normobaric wind tunnels and maximal treadmill running under hypoxia levels comparable to Mount Everest’s summit (∼7 kPa) (18, 31a). This impressive ability to sustain high metabolic rates in oxygen-poor air is attributed to enhancements in several steps of the oxygen transport pathway, boosting cellular Po2 (FIGURE 3).
FIGURE 4.
Figure 4: Enhanced cardiorespiratory and metabolic responses to hypoxia in bar-headed geese compared to lowland waterfowl. Total ventilation (V̇Tot) and cardiac output (Q̇) increase in hypoxia before blood oxygen desaturation, with a much greater increase in bar-headed geese than greylag geese. This heightened cardiorespiratory response is associated with a larger increase in oxygen consumption rate (V̇o2), shown by bar areas in right panels for each species in normoxia and 5% inspired O2. Data from Ref. 41, cardiac output calculated by Fick equation from Ref. 41 data.
The control of breathing in bar-headed geese has evolved to optimize oxygen uptake in hypoxic conditions. They exhibit larger increases in total ventilation in response to severe hypoxia (inspired Po2 ≤6 kPa) than any other bird species studied (5, 41, 42). For instance, total ventilation in bar-headed geese during severe hypoxia is approximately double that of the greylag goose, a closely related species that typically flies at lower altitudes (FIGURE 4). Furthermore, bar-headed geese breathe more deeply (with higher tidal volumes) and less frequently than low-altitude birds at the same level of total ventilation (39, 41). This breathing pattern is more effective for gas exchange, reducing dead-space ventilation and resulting in higher Po2 at the lung’s gas-exchange surface (compare FIGURE 3, A AND B). Bar-headed geese also have around 25% larger lungs compared to lowland waterfowl of similar body mass (45), increasing the area and diffusion capacity of the pulmonary gas-exchange surface. These adaptations enable bar-headed geese to maintain higher Po2 in their arterial blood than lowland counterparts during hypoxia (compare FIGURE 3 C AND D; FIGURE 4).
Circulatory oxygen delivery in hypoxia is improved in bar-headed geese through evolved changes in blood physiology. Their hemoglobin has a higher affinity for oxygen (whole-blood P50 of 4.0 kPa at pH 7.4 and CO2 tension of ∼5 kPa) than that of closely related lowland geese (5.3 kPa in greylag geese under the same conditions) (32). This increased affinity enhances pulmonary oxygen loading and peripheral oxygen delivery in hypoxia by increasing hemoglobin saturation at a given blood Po2 (43). The genetic basis for this higher affinity involves amino-acid substitutions in the hemoglobin protein’s α-subunit. Birds have major (HbA) and minor (HbD) hemoglobin forms. In bar-headed geese, the α-subunits of these forms contain four (αA) and two (αD) derived substitutions, respectively (30). Site-directed mutagenesis studies have shown that one substitution in αA (proline-119 → alanine) accounts for much of the increased oxygen affinity (23), likely by altering interactions between α- and β-subunits and destabilizing the protein’s deoxygenated state (57). Hemoglobin-oxygen binding in bar-headed geese is also more sensitive to temperature than in other birds and mammals (31). This could be significant for oxygen transport if there are temperature differences between lungs and flight muscles during flight (27, 43). For instance, blood warming in active flight muscles (8) would temporarily reduce hemoglobin oxygen affinity, promoting oxygen unloading. The benefit of this mechanism depends on the extent of thermal heterogeneity, which is currently unknown. Theoretical analyses suggest a 10°C temperature difference between lungs and flight muscles could increase oxygen transport in hypoxia by ∼40–60%, an effect amplified by the enhanced thermal sensitivity of bar-headed goose hemoglobin (43).
Evolved changes in heart function may also enhance circulatory oxygen delivery in hypoxic bar-headed geese. They have a 30–40% higher capillary density in the left ventricle of their heart than closely related lowland geese, but similar myoglobin concentration and maximal activity of several metabolic enzymes (e.g., citrate synthase, hydroxyacyl-coA dehydrogenase, lactate dehydrogenase, pyruvate kinase) (34, 45). This increased capillary density should raise Po2 in cardiac myocytes, improve heart hypoxemia tolerance, and allow bar-headed geese to increase cardiac output during hypoxia (FIGURE 4).
Flight muscle capillarity is also higher in bar-headed geese compared to lowland waterfowl (40), enhancing oxygen diffusion capacity from blood in hypoxia. Furthermore, a larger proportion of mitochondria in oxidative muscle fibers are located subsarcolemmally (near the cell membrane) in bar-headed geese (∼50%) versus lowland geese (∼35%) (40). This reduces intracellular oxygen diffusion distances. Each of these evolved specializations increases the capacity for extracting oxygen from the blood (compare FIGURE 3, E AND F) and maintaining high mitochondrial Po2 (compare FIGURE 3, G AND H) during high-altitude flight.
The enhanced oxygen transport capacity in hypoxic bar-headed geese (FIGURE 3) is accompanied by evolved changes in metabolic oxygen utilization in flight muscles and the heart. The proportion of oxidative fibers in flight muscles is higher in bar-headed geese (∼70% vs. ∼60% by area in the superficial pectoralis) compared to lowland waterfowl (40). However, mitochondrial respiratory capacity, oxygen kinetics (sensitivity to low oxygen tension), and mitochondrial abundance in oxidative fibers are similar in both groups (40). The affinity of cytochrome c oxidase (COX)—the enzyme consuming oxygen in oxidative phosphorylation—for cytochrome c is also higher in bar-headed geese (45). This change may result from a single mutation in COX subunit 3 at a conserved vertebrate site (tryptophan-116 → arginine), potentially altering inter-subunit interactions (45). The physiological significance of this trait is not fully understood, but it may reduce mitochondrial reactive oxygen species (ROS) production by allowing the electron transport chain to operate in a less reduced state. If so, this could lessen oxidative stress in bar-headed geese, a stressor associated with prolonged migration (22).
Mechanisms also appear to better match cellular ATP supply and demand in bar-headed goose flight muscles. Mitochondrial respiration in situ in permeabilized muscle fibers is more strongly regulated by creatine in bar-headed geese compared to low-altitude waterfowl (44). This suggests improved coupling of ATP supply and demand via the creatine kinase shuttle, crucial for intracellular ATP-equivalent transport (2). This trait might relate to subsarcolemmal mitochondrial localization in flight muscles, compensating for increased distance between these organelles and contractile elements.
Conclusion: Geese Soaring to Unprecedented Heights
High-altitude flight represents an extraordinary performance feat, underpinned by specialized physiological adaptations. Bar-headed geese achieve remarkable altitudes during Himalayan and Tibetan Plateau migrations because they can sustain the metabolic costs of flight even as air becomes extremely hypoxic. Like other migrating birds, they sometimes utilize updraft wind assistance to reduce flight costs. However, they also endure periods of intense flapping flight requiring extremely high heart rates, wing-beat frequencies, and metabolic power, such as during level high-elevation flight or climbs unaided by wind. Physiological specializations have evolved across the oxygen cascade in bar-headed geese, aiding this feat by enhancing oxygen transport in hypoxia. However, much of our understanding of bar-headed goose physiology comes from comparisons with lowland birds in sea-level environments. The discovered specializations likely do not fully explain their high-altitude capabilities. For example, we understand more about their hypoxia coping mechanisms than their responses to low barometric pressure, cold, and dry air at high altitudes. We also have limited knowledge about the influence of phenotypic plasticity (acclimatization) and developmental plasticity on their physiology. Much remains to be learned about the migration of these fascinating birds, which will undoubtedly continue to illuminate nature’s impressive solutions to oxygen deprivation.
Footnotes
We extend our gratitude to all field team members and acknowledge additional field work support from the Mongolian Academy of Sciences, Wildlife Science and Conservation Centre, Max Planck Institute for Ornithology, U.S. Geological Survey, Western Ecological and Patuxent Wildlife Research Centres and Avian Influenza Programme, United Nations Food and Agriculture Organization, and Beaumaris Instruments.
Research support was provided by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants to G. R. Scott and W. K. Milsom, and a UK Biotechnology and Biological Sciences Research Council (BBSRC) award to C. M. Bishop and P. J. Butler (grant no. BB/FO15615/1).
The authors declare no conflicts of interest, financial or otherwise.
Author contributions: G.R.S., L.A.H., and C.M.B. prepared figures; G.R.S. drafted manuscript; G.R.S., L.A.H., P.B.F., P.J.B., C.M.B., and W.K.M. edited and revised manuscript; G.R.S., L.A.H., P.B.F., P.J.B., C.M.B., and W.K.M. approved final manuscript version.
References
[1]: An, D., Ni, H., Zhang, W., and Zhang, Z. J. (2009). Opposing effects of hypocapnia on cerebral blood flow in rats and mice: role of BKCa channels. Am J Physiol Regul Integr Comp Physiol 297, R1710–R1718. https://doi.org/10.1152/ajpregu.00352.2009
[2]: Bakker, A. J., and Westerhoff, H. V. (2017). Creatine kinase: mechanism and function of the ubiquitous energy buffer. Biochemistry 56, 5615–5628. https://doi.org/10.1021/acs.biochem.7b00998
[3]: Bishop, C. M. (1997). Heart mass and stroke volume in birds in relation to body mass and mode of flight. J Exp Biol 200, 2785–2793.
[4]: Bishop, C. M., Butler, P. J., Egginton, S., and Jones, D. R. (2002). The cardiovascular system of bar-headed geese during flight at sea level and simulated altitude. J Exp Biol 205, 2743–2750.
[5]: Bishop, C. M., Green, J. A.,ອດEgginton, S., O’Malley, E. P., and Butler, P. J. (2015). Physiological responses to hypoxia in bar-headed geese and lowland waterfowl. J Exp Biol 218, 3735–3743. https://doi.org/10.1242/jeb.128387
[6]: Burger, R. E., and Meyer, M. (1990). Pulmonary circulation in birds and mammals. In Advances in Veterinary Science and Comparative Medicine, Vol. 34, edited by J. E. West. San Diego: Academic Press, pp. 1–49.
[7]: Butler, P. J. (2011). Flying at altitude: bar-headed geese and other high-altitude migrants. J Exp Biol 214, 2977–2985. https://doi.org/10.1242/jeb.050490
[8]: Butler, P. J., West, T. G., and Jones, D. R. (2004). Respiratory and cardiovascular systems: matching gas exchange to metabolic demand. In Avian Physiology, 5th ed., edited by P. D. Sturkie. San Diego: Academic Press, pp. 209–248.
[9]: Chai, P., Dillon, M. E., Wang, L., and Dudley, R. (2020). Aerodynamic and energetic benefits of tailwind assistance in avian flight. Integr Comp Biol 60, 1378–1393. https://doi.org/10.1093/icb/icaa101
[10]: Clausen, G., and Boutilier, R. G. (1989). pH regulation in exercising toads (Bufo marinus). Am J Physiol Regul Integr Comp Physiol 256, R859–R865. https://doi.org/10.1152/ajpregu.1989.256.4.R859
[11]: Deakin, J. E., and O’Halloran, F. (2000). Water loss in flying birds. Nature 407, 586. https://doi.org/10.1038/35036650
[12]: Faraci, F. M. (2011). Cerebral circulation: adaptations to high-altitude hypoxia. High Alt Med Biol 12, 193–200. https://doi.org/10.1089/ham.2010.1104
[13]: Faraci, F. M., and Parkinson, M. D. (1986). Pulmonary vascular responses to hypoxia in chickens. Am J Physiol Heart Circ Physiol 250, H1015–H1020. https://doi.org/10.1152/ajpheart.1986.250.6.H1015
[14]: Farmer, C. G. (2006). The evolution of air breathing in vertebrates: a late Cambrian origin. Integr Comp Biol 46, 1196–1215. https://doi.org/10.1093/icb/icl040
[15]: Frike, H., and Lissmann, H. W. (1964). Heart-rate and body temperature in flying birds. Nature 202, 1238–1239. https://doi.org/10.1038/2021238a0
[16]: Hawkes, L. A., Balachandran, S., Batbayar, N., Butler, P. J., Frappell, P. B., Milsom, W. K., et al. (2011). The paradox of extreme altitude migration in bar-headed geese (Anser indicus). Proc R Soc B Biol Sci 278, 3415–3421. https://doi.org/10.1098/rspb.2011.0977
[17]: Hawkes, L. A., Butler, P. J., Frappell, P. B., Jenni, E., Milsom, W. K., and Bishop, C. M. (2013). The metabolic challenges of high-altitude flight in bar-headed geese: flight speed and climb rate affect heart rate and estimated power consumption. J Exp Biol 216, 2127–2134. https://doi.org/10.1242/jeb.081248
[18]: Hogan, C. J., Bishop, C. M., Scott, G. R., and Milsom, W. K. (2017). Running at extreme altitude: bar-headed geese maintain maximal metabolic rate and exercise capacity under severe hypoxia. J Exp Biol 220, 1033–1042. https://doi.org/10.1242/jeb.151413
[19]: Houston, C. S. (1987). Cerebral edema at high altitude. N Engl J Med 317, 1530–1532. https://doi.org/10.1056/NEJM198712103172405
[20]: Kerlinger, P. (1995). How Birds Migrate. Mechanicsburg, PA: Stackpole Books.
[21]: Maina, J. N. (2002). The avian lung-air sac system: a paradigm of physiological and structural integration. J Exp Biol 205, 2245–2265.
[22]: Monaghan, P., Jennions, M. D., and Nunn, C. L. (2021). Oxidative stress, aging, and life histories: integrating ecology and evolution. Annu Rev Ecol Evol Syst 52, 211–235. https://doi.org/10.1146/annurev-ecolsys-011921-023848
[23]: Natarajan, C., Weber, R. E., Snyder, G. K., and Jensen, F. B. (2016). A single amino acid substitution in haemoglobin enhances flight performance of bar-headed geese at extreme altitude. J Exp Biol 219, 1407–1415. https://doi.org/10.1242/jeb.133445
[24]: Newman, S. H., and Bishop, C. M. (2015). How high can geese fly? An integrated approach to understanding bar-headed goose migration. Integr Comp Biol 55, 843–854. https://doi.org/10.1093/icb/icv070
[25]: Nilsson, G. E., and Lutz, P. L. (1991). Tolerance of anoxia and extreme hypoxia: vertebrates. Annu Rev Physiol 53, 545–562. https://doi.org/10.1146/annurev.ph.53.030191.002553
[26]: Nylander, E., and Swenson, E. R. (2014). Pulmonary hypertension in high-altitude illness. High Alt Med Biol 15, 279–286. https://doi.org/10.1089/ham.2014.1505
[27]: O’Dea, R. E., Frappell, P. B., and Butler, P. J. (2006). The role of temperature in avian respiratory gas exchange. J Exp Biol 209, 3527–3535. https://doi.org/10.1242/jeb.02410
[28]: Piiper, J., and Scheid, P. (1972). Respiration: rate and depth of breathing. In Physiology of Respiration, edited by J. H. Comroe Jr. Chicago: Year Book Medical Publishers, pp. 121–149.
[29]: Mathieu-Costello, O., Agey, P. J., and Weibel, E. R. (1989). Capillaries and mitochondria in muscle fibers of hummingbirds compared with rat and dog. Am J Physiol Cell Physiol 257, C334–C343. https://doi.org/10.1152/ajpcell.1989.257.2.C334
[30]: Peterson, J. D., Lu, Y., Haigh, J. J., Scott, G. R., and Storz, J. F. (2021). Evolution of adaptive variation in hemoglobin function: insights from comparative genomics and phylogeography of bar-headed geese. Philos Trans R Soc B Biol Sci 376, 20200378. https://doi.org/10.1098/rstb.2020.0378
[31]: Rummer, J. L., Isaacks, A., Kennedy, R. B., and Brauner, C. J. (2016). The functional significance of temperature-sensitive haemoglobin-oxygen binding in bar-headed geese (Anser indicus). J Exp Biol 219, 2535–2542. https://doi.org/10.1242/jeb.139308
[31a]: Scott, G. R., Hogan, C. J., Natarajan, C., Poole, R. K., Nilsen, R., Reid, S. G., et al. (2011). Probing the limits to aerobic performance: bar-headed geese during maximal exercise at extreme altitude. J Exp Biol 214, 2964–2969. https://doi.org/10.1242/jeb.058220
[32]: Scott, G. R., Milsom, W. K., Kvist, A., Weber, R. E., and Rees, S. E. (2008). Evolution of hemoglobin function in high-altitude Andean geese. Am J Physiol Regul Integr Comp Physiol 295, R195–R203. https://doi.org/10.1152/ajpregu.90195.2008
[33]: Scheid, P., and Piiper, J. (1972). Cross-current gas exchange in avian lungs: models and experimental evidence. Respir Physiol 16, 304–315. https://doi.org/10.1016/0034-5687(72)90096-1
[34]: Semenza, G. L. (2014). Oxygen sensing, homeostasis, and disease. N Engl J Med 371, 537–548. https://doi.org/10.1056/NEJMra1402283
[35]: Severinghaus, J. W. (1999). Physiological rationale for protection of altitude natives against chronic mountain sickness. High Alt Med Biol 0, 171–182. https://doi.org/10.1089/ham.1999.0.171
[36]: Maina, J. N. (2006). The Avian Respiratory System. Berlin: Springer.
[37]: Swenson, E. R. (2013). High-altitude pulmonary edema. High Alt Med Biol 14, 101–110. https://doi.org/10.1089/ham.2012.1097
[38]: Storz, J. F., and Scott, G. R. (2019). Physiological adaptation to life at high altitude. Compr Physiol 9, 1247–1291. https://doi.org/10.1002/cphy.c180040
[39]: Snyder, G. K. (1991). Adaptations of birds to high altitude. Am Zool 31, 506–516. https://doi.org/10.1093/icb/31.3.506
[40]: Snyder, G. K., Bishop, C. M., Black, C. P., and Hopper, D. L. (2004). Muscle fiber types and mitochondrial distribution in the flight muscle of bar-headed geese and greylag geese. J Exp Biol 207, 3737–3745. https://doi.org/10.1242/jeb.01203
[41]: Snyder, G. K., Black, C. P., and Cruz-Neto, A. P. (1998). Cardiorespiratory responses to hypoxia in bar-headed and greylag geese. J Exp Biol 201, 3275–3285.
[42]: Snyder, G. K., and Faraci, F. M. (2011). Cerebral blood flow and hypocapnia in birds and mammals. Respir Physiol Neurobiol 178, 167–171. [https://doi.org/10.1016/j.resp